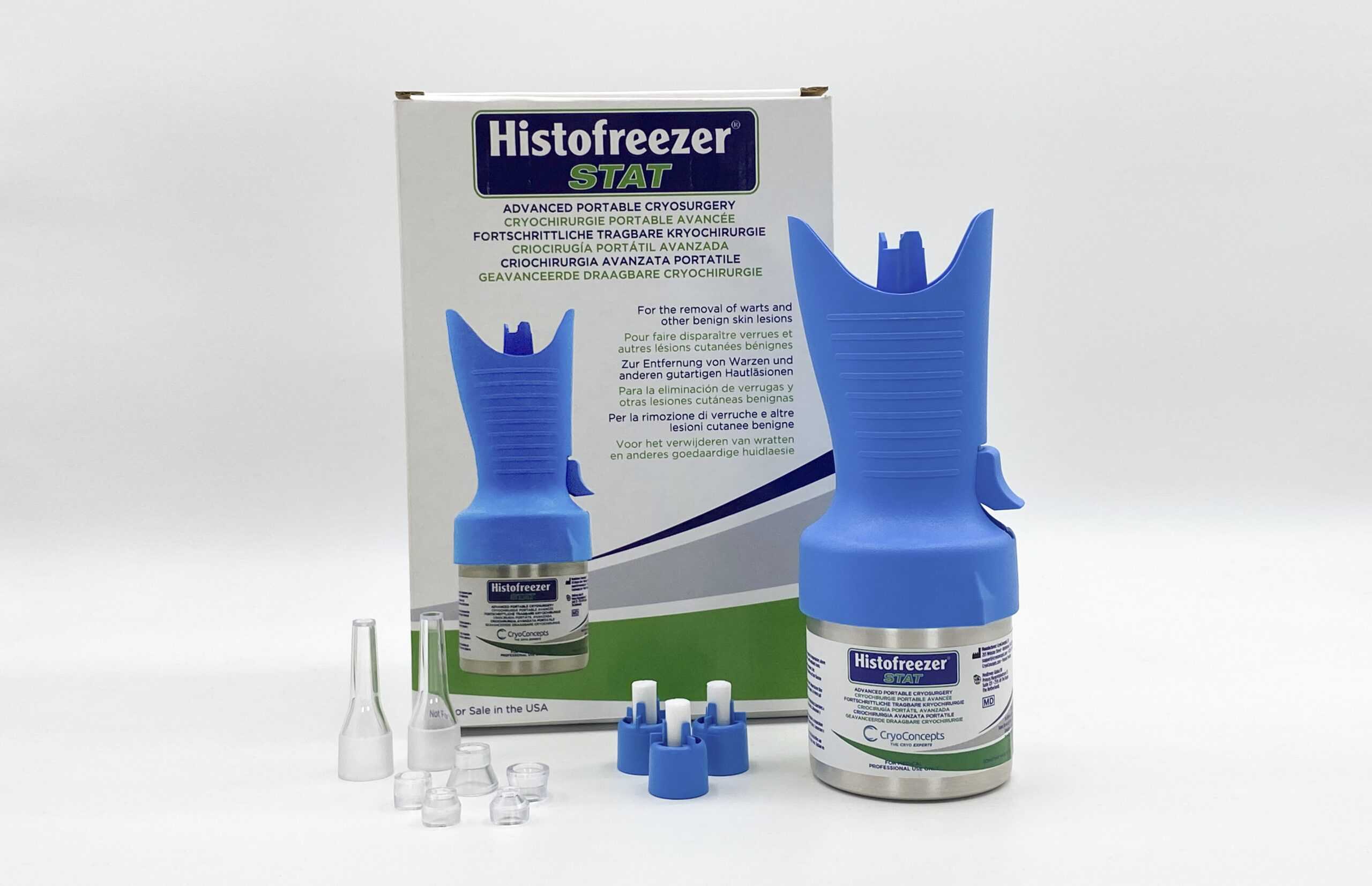
Environmentally Friendly Design Features Reusable Canisters
Histofreezer® continues to be a trusted choice by thousands of doctors to treat common and plantar warts for almost 30 years. Our cryosurgical device is easy-to-operate, safe for staff and patients, and it is highly effective.
Now, new Histofreezer® STAT — the first major advancement in portable cryosurgery in decades – is being introduced to the Canadian healthcare market in July, 2023.
Histofreezer® STAT works with cones or buds, making it easy to customize the treatment based on the patient’s needs. Both treatment options deliver a colder treatment which result in better first-time outcomes.
Histofreezer® STAT is the most environmentally-friendly cryosurgical device on the market. Our new cryogen has the lowest impact of any gas mixture and our canisters are reusable. So now, empty canisters are returned and refilled, which means they do not end up in a land fill.
New Histofreezer® STAT. Better design. Better outcomes. Better for the Environment.